|
|
|
发布时间: 2021-04-25 |
清洁安全发电 |
|
|
|
收稿日期: 2019-05-20
基金项目: 国家自然科学基金(21776172)
中图法分类号: O648.2
文献标识码: A
文章编号: 2096-8299(2021)02-0158-07
|
摘要
光催化技术和膜分离技术是两种新的水处理技术,二者耦合后形成的光催化膜不仅可以起到固定催化剂、缓解膜污染的作用,还可产生协同效应、提高水中污染物的降解效率。综述了光催化剂和负载膜的种类,以及光催化负载膜的研究现状。
关键词
光催化; 膜分离; 负载型光催化膜
Abstract
photocatalysis and membrane separation are two emerging technologies in water treatment technology.By coupling the two technologies, the photocatalytic membrane formed can not only fix the catalyst and alleviate membrane pollution, but also produce synergistic effect and improve the degradation efficiency of pollutants in water.The types of photocatalyst and supporting film and the research status of supporting film for photocatalysis are reviewed.
Key words
photocatalytic; membrane separation; supported photocatalytic membrane
随着工业的进步和科技的发展, 人民生活水平的不断提高, 环境问题也逐步凸显出来, 从源头减少污染和完善污染治理措施是当前面临的严峻问题。在全球地表水水质不断恶化和饮用水水质标准日益严格的大背景下, 水资源的梯级利用和污水回用是解决水资源短缺等问题的重要途径。但随着工业企业的不断增多, 污水的成分也越来越复杂, 如染料废水、医疗废水、农田径流雨水、制药废水等。成分复杂的污染物排放, 不仅阻碍了水资源的充分利用和污水回用, 而且会对人类和水生动物造成严重的危害。
如今, 传统的污水处理方法(如活性污泥法)对成分复杂污染物的降解效果已经不能满足要求。高级氧化技术也是一种常见的污水处理技术, 是通过紫外线和臭氧及H2O2等氧化物将污染物氧化成CO2和H2O及无机小分子[1]。该技术具有降解效率高、出水水质好等优点, 但在实际工业使用中存在能耗高、成本高等缺点。光催化氧化技术可以有效地降解多种成分复杂的污染物, 在污水体系中, 光催化剂经光照后产生的羟基自由基和氧负离子会将污染物矿化成CO2和H2O及一些无机离子。已有实验研究表明, 光催化技术能够较好地降解水中的抗生素[2]、细胞抑制剂[3]、染料[4]、农药[5]、内分泌干扰素[6]、大肠杆菌[7]等。由于光催化氧化技术是利用光源对污染物尤其是有机物进行降解, 因此在光催化过程中无二次污染物, 且无毒、能耗低、运行费用少[8]。
污水处理中的另一种技术是膜分离。膜分离技术是指在外加压力的作用下, 利用膜材料自身的选择透过性, 将水中不同组分进行分离的过程[9]。膜分离是一个完全的物理过程, 并不涉及污染物的降解。将膜分离与光催化结合, 二者的协同作用便能够显现出来。光催化剂能够有效地降解膜表面沉积的污染物, 有效缓解膜污染, 而膜能够固定住光催化剂, 使光催化剂可以与污染物充分接触, 达到降解污染物的效果。
1 光催化剂
1.1 作用机理
FUJISHIMA A等人[10]于1972年发表了一篇关于N型半导体材料分解水实验的文章, 使得半导体光催化材料受到了广泛的关注。包括气相、液相中有机物和无机物的降解、产氢以及光致还原CO2等。
光催化剂, 其本质是一类半导体材料, 其价带(VB)和导带(CB)之间存在能带隙。当半导体表面受到光照的能量大于其能带隙的能量时, 价带的电子被激发, 跃迁到导带, 产生电子空穴(h+), 被激发的电子叫做光生电子(e-)。光生电子和空穴对迁移到半导体材料的表面, 可以与其他物质反应, 表现出还原性和氧化性[11]。在水处理中, 应用较多的是电子空穴的氧化性。由于价带的氧化性高于一般有机物的氧化性, 因而生成具有高度氧化活性的羟基自由基和超氧自由基[12-13]。
1.2 研究现状
常见的光催化剂有TiO2, g-C3N4, ZnO, Ag3PO4, CdS, CuO, ZnS, CuWO4, VS4, V2O5, Cu2O, 以及Bi系光催化剂等[14-24]。其中, 应用最为广泛的是TiO2[25]。
TiO2是一类N型半导体材料, 有板钛矿型、金红石矿型和锐钛矿型3种晶型[26]。在紫外光(波长<385 nm)的照射下, 价带的电子被激发, 跃迁到导带(其中锐钛矿的禁带宽度为3.2 eV, 金红石矿的禁带宽度为3.0 eV)。当锐钛矿型TiO2与金红石矿的TiO2比为4∶ 1时, 其光催化活性最佳[27]。TiO2之所以得到广泛应用, 主要是因为其价格低、无毒且物理化学性质稳定, 对光照的吸收稳定且不产生光辐射, 在紫外光的照射下可以展现出良好的光催化活性。WANG X等人[28]将N和P共掺杂的TiO2负载到膨胀石墨烯上, 制成了一种浮在水面上的碳层复合材料。这种光催化材料可以有效利用光源, 且易于回收。实验结果表明, 复合光催化膜对微囊藻素的去除率可达到99.4%。
此外, 不含金属元素的石墨相氮化碳(g-C3N4)因其独特的层状结构也引起了研究者的注意[29]。g-C3N4也是一类非金属聚合物半导体材料, 禁带宽度为2.7 eV(价带宽度为1.4 eV, 导带宽度为-1.3 eV), 可以被460 nm的光激发, 产生光生电子和电子空穴, 具有光催化活性, 可用于降解污水中的污染物、光解水产氢、还原CO2及消毒等[30-33]。g-C3N4具有成本低、毒性低、环境友好以及能响应可见光等特点[34], 但由于其本质是一类高分子材料, 量子效率相对较低, 氧化还原电位较低, 故限制了g-C3N4的应用。ZHAO H等人[35]利用真空抽滤和高压工艺将g-C3N4负载的氧化石墨烯纳米片负载到醋酸纤维素膜上, 得到的g-C3N4/RGO复合醋酸纤维素膜展现出较强的染料去除率和抗菌性。
ZnO是一类N型半导体材料, 其禁带宽度为3.2 eV, 具有形态多样、电子转移效率高、价格低、对环境无污染等优点[36]。ZnO的制备条件不同, 材料的结晶性和比表面积也不相同, 进而影响其光催化活性。ZnO因其具有形态易控制的优点被广泛应用。CANTARELLA M等人[37]利用共沉淀法制备ZnO, 在制备过程中加入了醋氨酚, 使ZnO的形态结构发生了变化, 使醋氨酚的吸附效率明显增强。
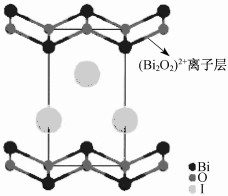
Bi系催化剂主要有BiVO4, Bi2WO6, Bi2MoO6, BiOBr, BiOI, BiFeO3, CaBi2O4, Bi2O3, Bi2S3, Bi2Ti2O7, BiOCl等[38-40]。其中, BiOI是一类P型半导体材料, 禁带宽度为1.8 eV(价带宽度为2.35 eV, 导带宽度为0.54 eV), 具有独特的层状结构。其晶体结构如图 1所示。BiOI是由两层[Bi2O2]2+离子层构成, 中间为Bi-离子层。由于BiOI的禁带宽度较窄, 因此对于可见光有明显的吸收作用, 但纯的BiOI对污染物的去除能力有限。
单一的Bi系光催化剂的降解效果并不能满足需要, 大多数研究是将两种光催化剂通过半导体复合的方法构建成异质结结构。HUANG H W等人[38]用水热法制备BiVO4, 用溶胶-凝胶法制备BiOI, 将BiVO4纳米粒与BiOI纳米片复合, 构建N-P型异质结结构, 降解罗丹明B和苯酚时, 显示出比单独使用BiVO4或BiOI降解时更好的效果。
Bi系催化剂也可以与其他催化剂复合, 构建异质结。如LI B等人[41]使用水热法制成BiOI/TiO2异质结。实验表明, BiOI/TiO2较单独的BiOI或TiO2有更优异的光催化性能, 且当BiOI与TiO2的摩尔比为1∶ 5时, 达到最高的污染物降解率。这是由于BiOI能与TiO2形成P-N异质结结构, 有效减小TiO2的禁带宽度, 暴露出更多的活性位点。
2 光催化膜
2.1 作用机理
2.2 研究现状
将光催化过程与膜分离技术相结合, 构成光催化膜系统。二者耦合不仅可以高效处理废水, 而且能够有效缓解膜分离带来的膜污染问题[48]。根据膜的孔径可分为超滤、纳滤、微滤、反渗透4种[49]。根据膜的材料可分为无机膜和有机膜[50-51]。
现阶段, 国内外的研究者开发了多种可以用于负载光催化剂的膜基底材料: 无机膜, 包括氧化铝、碳化硅等; 金属网格材料, 包括钛网、铜网等; 玻璃纤维; 碳纤维; 高分子聚合物膜; 纤维素及其衍生物, 如醋酸纤维素、硝酸纤维素等。
无机膜具有耐酸碱、耐高温、化学性质稳定、机械强度高、易清洗等优点[52], 因此在石油、工业、食品等领域被广泛应用。无机膜是由无机材料如氧化铝、氧化锆、碳化硅、二氧化钛等添加适量的添加剂在高温下煅烧而成的。其主要结构由支撑层、过渡层和膜层3部分组成。将光催化剂负载到陶瓷膜上, 不仅可以降解污染物, 还可以有效缓解膜污染。ZHANG Q等人[53]用浸渍-涂覆法, 将TiO2纳米纤维负载至中空纤维陶瓷膜表面, 负载膜对腐殖酸的去除率可达90%。这证明将光催化剂负载到无机膜表面, 可使无机膜具有自清洁功能, 有效缓解膜污染。这种自清洁膜也已应用于实际的分离和提纯工艺等方面[54]。但由于无机膜的制备过程中需要高温煅烧, 使得其成本较高, 且实验中重复使用次数较少, 因此限制了其广泛应用。
金属网格材料具有均匀、致密的网格结构, 良好的化学稳定性、热稳定性和机械性能。LIN Y Q等人[55]将TiO2负载到Ti膜上, 得到的光催化膜中催化剂与膜克服了传统的陶瓷光催化膜中催化剂与膜热膨胀系数不匹配的情况, 且具有良好的光催化降解染料的性能。QIAN D L等人[56]将银离子、磺化的氧化石墨烯及TiO2分别负载到铜网上, 所制备的铜网具有双向排斥性, 能有效分离水油乳浊液。而且在降解过程中, 致密的TiO2簇负载在铜网上, 能够预防铜被腐蚀和氧化。金属网格材料在工业使用过程中可通过焊接等方式固定。因此, 这种金属网格光催化膜具有良好的实际应用前景。
玻璃纤维通常由SiO2制成, 具有良好的紫外光透过性。相对其他膜材料, 利用玻璃纤维制成的光催化膜有更大的光线接触面积, 从而具有更好的催化活性。RAO G Y等人[57]合成了TiO2/Fe2O3/GO负载玻璃纤维膜, 用于降解腐殖酸, 2 h紫外光照射下腐殖酸的降解效率为98%。
碳纤维是一种常见的材料, 已广泛应用于各种催化剂的支撑体。碳纤维材料用于光催化膜不仅可以增大催化剂的比表面积, 而且具有良好的力学性能、导电性能和抗腐蚀性能[23]。SHEN X F等人[58]制备了过滤型的氮化碳复合碳纳米纤维滤膜, 对罗丹明B染料模拟废水60 min的降解率可达到98%。
高分子聚合物膜的种类有很多, 大致可分为聚烯烃类[59]、聚酰胺类[60]、聚砜类[61-62]和含氟高分子材料[63]等。这类光催化膜通常采用界面聚合或相转化的方法制备, 制成的复合膜具有化学稳定性和热稳定性好, 膜孔道丰富, 光催化剂不易脱落等优点。YU S等人[62]通过相转化方法制备了g-C3N4/TiO2复合聚砜膜, 降解磺胺甲恶唑模拟医药废水。实验结果表明, 复合聚砜膜可以将磺胺甲恶唑成功降解成7种中间产物。但是高分子聚合物膜的成本相对较高, 且膜的重复使用性和持久性较差。
纤维素及其衍生物(如醋酸纤维素、硝酸纤维素等)是比较常见的一种实验室用滤膜, 其生产工艺成熟, 且价格低廉, 易于获得。在过滤方面, 热稳定性较好, 吸附量较低。LI F等人[64]用聚多巴胺改性RGO/g-C3N4复合光催化剂, 再用真空抽滤的方法将复合的催化剂负载到醋酸纤维素滤膜上。该复合膜对亚甲基蓝染料废水有很高的截留效率, 在紫外光照射下, 循环使用5次后仍具备良好的光催化活性。此类纤维素滤膜不仅可以实现牢固负载, 保持光催化剂的光催化活性, 而且原料易得, 制备过程简单。MOHAMED M A等人[65]利用旧报纸作为纤维素原材料, 通过相转化法制备了N改性的TiO2复合醋酸纤维素滤膜, 用于降解苯酚废水, 取得了良好的实验结果。
3 结语
可同时用于膜分离和光催化的负载型光催化膜种类有很多, 且复合膜在水处理方面的应用潜力巨大, 但仍有很多亟待解决的问题, 如使光催化剂能够牢固负载的制备工艺, 高效稳定的膜材质的研发和光催化膜分离二者作用的耦合机理等。随着环境问题的日益严峻, 多功能的光催化膜在污染物质的降解和污水回用等领域具有广阔的发展前景。
参考文献
-
[1]孙怡, 于利亮, 黄浩斌, 等. 高级氧化技术处理难降解有机废水的研发趋势及实用化进展[J]. 化工学报, 2017, 68(5): 1743-1756.
-
[2]KARAOLIA P, MICHAEL-KORDATOU I, HAPESHI E, et al. Removal of antibiotics, antibiotic-resistant bacteria and their associated genes by graphene-based TiO2 composite photocatalysts under solar radiation in urban wastewaters[J]. Applied Catalysis B: Environmental, 2017(11): 810-824.
-
[3]JANSSENS R, MANDAL M K, DUBEY K K, et al. Slurry photocatalytic membrane reactor technology for removal of pharmaceutical compounds from wastewater: towards cytostatic drug elimination[J]. Science of the Total Environment, 2017(3): 612-626.
-
[4]VILLABONA-LEAL E G, LÓPEZ-NEIRA J P, PEDRAZA-AVELLA J A, et al. Screening of factors influencing the photocatalytic activity of TiO2: Ln (Ln=La, Ce, Pr, Nd, Sm, Eu and Gd) in the degradation of dyes[J]. Computational Materials Science, 2015(5): 48-53.
-
[5]BERBERIDOU C, KITSIOU V, LAMBROPOULOU D A, et al. Evaluation of an alternative method for wastewater treatment containing pesticides using solar photocatalytic oxidation and constructed wetlands[J]. Journal of Environment Manage, 2017, 195(2): 133-139.
-
[6]MIRZAEI A, CHEN Z, HAGHIGHAT F, et al. Removal of pharmaceuticals and endocrine disrupting compounds from water by zinc oxide-based photocatalytic degradation: a review[J]. Sustainable Cities and Society, 2016(8): 407-418.
-
[7]ZHANG Y, LIN C, LIN Q, et al. CuI-BiOI/Cu film for enhanced photo-induced charge separation and visible-light antibacterial activity[J]. Applied Catalysis B: Environmental, 2018(5): 238-245.
-
[8]WANG H, YUAN X, WU Y, et al. Synthesis and applications of novel graphitic carbon nitride/metal-organic frameworks mesoporous photocatalyst for dyes removal[J]. Applied Catalysis B: Environmental, 2015(3): 445-454.
-
[9]PHAN D D, BABICK F, TRINH T H T, et al. Investigation of fixed-bed photocatalytic membrane reactors based on submerged ceramic membranes[J]. Chemical Engineering Science, 2018(6): 332-342.
-
[10]FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238: 37. DOI:10.1038/238037a0
-
[11]MOLINARI R, LAVORATO C, ARGURIO P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds.a review[J]. Catalysis Today, 2016, 6: 144-164.
-
[12]SHEHZAD N, TAHIR M, JOHARI K, et al. A critical review on TiO2 based photocatalytic CO2 reduction system: strategies to improve efficiency[J]. Journal of CO2 Utilization, 2018, 26(4): 98-122.
-
[13]GUO B, SNOW S D, STARR B J, et al. Photocatalytic inactivation of human adenovirus 40:effect of dissolved organic matter and prefiltration[J]. Separation and Purification Technology, 2017(11): 193-201.
-
[14]JIANG D, XUE J, WU L, et al. Photocatalytic performance enhancement of CuO/Cu2O heterostructures for photodegradation of organic dyes: effects of CuO morphology[J]. Applied Catalysis B: Environmental, 2017(4): 199-204.
-
[15]HONG Y, JIANG Y, LI C, et al. In-situ synthesis of direct solid-state Z-scheme V2O5/g-C3N4 heterojunctions with enhanced visible light efficiency in photocatalytic degradation of pollutants[J]. Applied Catalysis B: Environmental, 2015(6): 663-673.
-
[16]LU H J, HAO Q, CHEN T, et al. A high-performance Bi2O3/Bi2SiO5 p-n heterojunction photocatalyst induced by phase transition of Bi2O3[J]. Applied Catalysis B: Environmental, 2018(5): 59-67.
-
[17]WANG X C, MAEDA K, THOMAS A, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light[J]. Nature Materials, 2008, 8: 76.
-
[18]CHEN W Y, NIU X J, WANG J. A photocatalyst of graphene oxide (GO)/Ag3PO4 with excellent photocatalytic activity over decabromodiphenyl ether (BDE-209) under visible light irradiation[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2017(12): 304-311.
-
[19]VAMVASAKIS I, PAPADAS I T, TZANOUDAKIS T, et al. Visible-light photocatalytic H2 production activity of β-Ni(OH)2-modified CdS mesoporous nanoheterojunction networks[J]. ACS Catalysis, 2018, 8/9: 8726-8738.
-
[20]ZHU C, LIU C, ZHOU Y, et al. Carbon dots enhance the stability of CdS for visible-light-driven overall water splitting[J]. Applied Catalysis B: Environmental, 2017(5): 114-121.
-
[21]BASHIRI R A, MONTAZER M, MAHMOUDI R M. Environmentally friendly low cost approach for nano copper oxide functionalization of cotton designed for antibacterial and photocatalytic applications[J]. Journal of Cleaner Production, 2018(8): 425-436.
-
[22]ZHANG B, ZOU S, CAI R, et al. Highly-efficient photocatalytic disinfection of Escherichia coli under visible light using carbon supported vanadium tetrasulfide nanocomposites[J]. Applied Catalysis B: Environmental, 2017(10): 383-393.
-
[23]CHEN H, JIANG G H, YU W J, et al. Preparation of electrospun ZnS-loaded hybrid carbon nanofiberic membranes for photocatalytic applications[J]. Powder Technol, 2016(5): 1-8.
-
[24]LIMA A E B, COSTA M J S, SANTOS R S, et al. Facile preparation of CuWO4 porous films and their photoelectrochemical properties[J]. Electrochim Acta, 2017(10): 139-145.
-
[25]LEI M, WANG N, ZHU L H, et al. Photocatalytic reductive degradation of polybrominated diphenyl ethers on CuO/TiO2 nanocomposites: a mechanism based on the switching of photocatalytic reduction potential being controlled by the valence state of copper[J]. Applied Catalysis B: Environmental, 2015(9): 414-423.
-
[26]HAIDER Z, KANG Y S. Facile preparation of hierarchical TiO2 nano structures: growth mechanism and enhanced photocatalytic H2 production from water splitting using methanol as a sacrificial reagent[J]. ACS Applied Materials and Interfaces, 2014, 6(13): 10342-10352. DOI:10.1021/am501796m
-
[27]HE Z Q, CAI Q L, FANG H Y, et al. Photocatalytic activity of TiO2 containing anatase nanoparticles and rutile nanoflower structure consisting of nanorods[J]. Journal of Environmental Sciences, 2013, 25(12): 2460-2468. DOI:10.1016/S1001-0742(12)60318-0
-
[28]WANG X, WANG X J, ZHAO J F, et al. An alternative to in situ photocatalytic degradation of microcystin-LR by worm-like N, P co-doped TiO2/expanded graphite by carbon layer (NPT-EGC) floating composites[J]. Applied Catalysis B: Environmental, 2017(1): 479-489.
-
[29]ONG W J, TAN L L, NG Y H, et al. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability?[J]. Chemical Reviews, 2016(12): 7159-7329.
-
[30]MA S, ZHAN S, JIA Y, et al. Enhanced disinfection application of Ag-modified g-C3N4 composite under visible light[J]. Applied Catalysis B: Environmental, 2015(12): 77-87.
-
[31]DE SILVA S W, DU A, SENADEERA W, et al. Strained graphitic carbon nitride for hydrogen purification[J]. Journal of Membrane Science, 2017(1): 201-205.
-
[32]LIU S, CHEN F, LI S, et al. Enhanced photocatalytic conversion of greenhouse gas CO2 into solar fuels over g-C3N4 nanotubes with decorated transparent ZIF-8 nanoclusters[J]. Applied Catalysis B: Environmental, 2017(4): 1-10.
-
[33]张金水, 王博, 王心晨. 氮化碳聚合物半导体光催化[J]. 化学进展, 2014, 26(1): 19-29.
-
[34]CUI Y. Insitu synthesis of C3N4/CdS composites with enhanced photocatalytic properties[J]. Chinese Journal of Catalysis, 2015, 36(3): 372-379. DOI:10.1016/S1872-2067(14)60237-0
-
[35]ZHAO H, CHEN S, QUAN X, et al. Integration of microfiltration and visible-light-driven photocatalysis on g-C3N4 nanosheet/reduced graphene oxide membrane for enhanced water treatment[J]. Applied Catalysis B: Environmental, 2016(4): 134-140.
-
[36]JIANG J J, WANG H T, CHEN X D, et al. Enhanced photocatalytic degradation of phenol and photogenerated charges transfer property over BiOI-loaded ZnO composites[J]. Journal of Colloid Interface Science, 2017(1): 130-138.
-
[37]CANTARELLA M, DI MAURO A, GULINO A, et al. Selective photodegradation of paracetamol by molecularly imprinted ZnO nanonuts[J]. Applied Catalysis B: Environmental, 2018(7): 509-517.
-
[38]HUANG H W, HE Y, DU X, et al. A general and facile approach to heterostructured core/shell BiVO4/BiOI p-n junction: room-temperature in situ assembly and highly boosted visible-light photocatalysis[J]. ACS Sustainable Chemistry & Engineering, 2015, 3(12): 3262-3273.
-
[39]YIN Y Y, LIU Q, JIANG D, et al. Atmospheric pressure synthesis of nitrogen doped graphene quantum dots for fabrication of BiOBr nanohybrids with enhanced visible-light photoactivity and photostability[J]. Carbon, 2015(10): 1157-1165.
-
[40]HE R A, CAO S, ZHOU P, et al. Recent advances in visible light Bi-based photocatalysts[J]. Chinese Journal of Catalysis, 2014, 35(7): 989-1007. DOI:10.1016/S1872-2067(14)60075-9
-
[41]LI B, CHEN X W, ZHANG T Y, et al. Photocatalytic selective hydroxylation of phenol to dihydroxybenzene by BiOI/TiO2 p-n heterojunction photocatalysts for enhanced photocatalytic activity[J]. Applied Surface Science, 2017(12): 1047-1056.
-
[42]BRUNETTI A, ZITO P F, GIORNO L, et al. Membrane reactors for low temperature applications: an overview[J]. Chemical Engineering and Processing-Process Intensification, 2017(5): 282-307.
-
[43]BRUNET E R, RAFIEIAN D, POSTMA R S, et al. Egg-shell membrane reactors for nitrite hydrogenation: manipulating kinetics and selectivity[J]. Applied Catalysis B: Environmental, 2017(10): 276-282.
-
[44]GHAFFAR A, ZHANG L, ZHU X Y, et al. Porous PVdF/GO nanofibrous membranes for selective separation and recycling of charged organic dyes from water[J]. Environ Science Technol, 2018, 52(7): 4265-4274. DOI:10.1021/acs.est.7b06081
-
[45]张宏忠, 张钰, 王明花, 等. 二氧化钛光催化膜分离耦合技术在水处理中的应用[J]. 无机盐工业, 2017, 49(7): 50-54.
-
[46]DU X, QU F S, LIANG H, et al. Control of submerged hollow fiber membrane fouling caused by fine particles in photocatalytic membrane reactors using bubbly flow: shear stress and particle forces analysis[J]. Separation and Purification Technology, 2016(8): 130-139.
-
[47]TAN Y Z, WANG H, HAN L, et al. Photothermal-enhanced and fouling-resistant membrane for solar-assisted membrane distillation[J]. Journal of Membrane Science, 2018(8): 254-265.
-
[48]JIANG L, ZHANG X L, CHOO K H. Submerged microfiltration-catalysis hybrid reactor treatment: photocatalytic inactivation of bacteria in secondary wastewater effluent[J]. Separation and Purification Technology, 2017(1): 87-92.
-
[49]ZHANG Y, WAN Y, PAN G, et al. Surface modification of polyamide reverse osmosis membrane with organic-inorganic hybrid material for antifouling[J]. Applied Surface Science, 2018(5): 139-148.
-
[50]WU X N, ZHAO B, WANG L, et al. Superhydrophobic PVDF membrane induced by hydrophobic SiO2 nanoparticles and its use for CO2 absorption[J]. Separation and Purification Technology, 2017(7): 108-116.
-
[51]LI J J, ZHOU Y N, LUO Z H. Polymeric materials with switchable superwettability for controllable oil/water separation: a comprehensive review[J]. Progress in Ploymer Science, 2018(6): 1-33.
-
[52]ALEM A, SARPOOLAKY H, KESHMIRI M. Titania ultrafiltration membrane: preparation, characterization and photocatalytic activity[J]. Journal of the European Ceramic Society, 2009, 29(4): 629-635. DOI:10.1016/j.jeurceramsoc.2008.07.003
-
[53]ZHANG Q, WANG H, FAN X, et al. Fabrication of TiO2 nanofiber membranes by a simple dip-coating technique for water treatment[J]. Surface and Coatings Technology, 2016(4): 45-52.
-
[54]ALIAS S S, HARUN Z, LATIF I S A. Characterization and performance of porous photocatalytic ceramic membranes coated with TiO2 via different dip-coating routes[J]. Journal of Materials Science, 2018, 53(16): 11534-11552. DOI:10.1007/s10853-018-2392-3
-
[55]LIN Y Q, CAI Y Y, DRIOLI E, et al. Enhancing mechanical and photocatalytic performances on TiO2/Ti composite ultrafiltration membranes via Ag doping method[J]. Separation and Purification Technology, 2015(2): 29-38.
-
[56]QIAN D L, CHEN D Y, LI N J, et al. TiO2/sulfonated graphene oxide/Ag nanoparticle membrane: in situ separation and photodegradation of oil/water emulsions[J]. Journal of Membrane Science, 2017(12): 16-25.
-
[57]RAO G Y, ZHANG Q Y, ZHAO H L, et al. Novel titanium dioxide/iron (Ⅲ) oxide/graphene oxide photocatalytic membrane for enhanced humic acid removal from water[J]. Chemical Engineering Journal, 2016(5): 633-640.
-
[58]SHEN X F, ZHANG T Y, XU P F, et al. Growth of C3N4 nanosheets on carbon-fiber cloth as flexible and macroscale filter-membrane-shaped photocatalyst for degrading the flowing wastewater[J]. Applied Catalysis B: Environmental, 2017(7): 425-431.
-
[59]MANTILAKA M M M G P G, DE SILVA R T, RATNAYAKE S P, et al. Photocatalytic activity of electrospun MgO nanofibres: synthesis, characterization and applications[J]. Materials Research Bulletin, 2017(10): 204-210.
-
[60]SU J F, YANG G H, CHENG C L, et al. Hierarchically structured TiO2/PAN nanofibrous membranes for high-efficiency air filtration and toluene degradation[J]. Journal of Colloid Interface Science, 2017(7): 386-396.
-
[61]ZANGENEH H, ZINATIZADEH A A, ZINADINI S, et al. A novel photocatalytic self-cleaning PES nanofiltration membrane incorporating triple metal-nonmetal doped TiO2 (K-B-N-TiO2) for post treatment of biologically treated palm oil mill effluent[J]. Reactive and Functional Polymers, 2018(4): 139-152.
-
[62]YU S, WANG Y, SUN F, et al. Novel mpg-C3N4/TiO2 nanocomposite photocatalytic membrane reactor for sulfamethoxazole photodegradation[J]. Chemical Engineering Journal, 2017(12): 183-192.
-
[63]PAREDES L, MURGOLO S, DZINUN H, et al. Application of immobilized TiO2 on PVDF dual layer hollow fibre membrane to improve the photocatalytic removal of pharmaceuticals in different water matrices[J]. Applied Catalysis B: Environmental, 2018(8): 9-18.
-
[64]LI F, YU Z, SHI H, et al. A mussel-inspired method to fabricate reduced graphene oxide/g-C3N4 composites membranes for catalytic decomposition and oil-in-water emulsion separation[J]. Chemical Engineering Journal, 2017(3): 33-45.
-
[65]MOHAMED M A, SALLEH W N W, JAAFAR J, et al. Physicochemical characteristic of regenerated cellulose/N-doped TiO2 nanocomposite membrane fabricated from recycled newspaper with photocatalytic activity under UV and visible light irradiation[J]. Chemical Engineering Journal, 2015(8): 202-215.